Page 229 - C_Modena
P. 229
Centro di Medicina Rigenerativa “Stefano Ferrari” | “Stefano Ferrari” Regenerative Medicine Centre 307
could answer to local companies’ requests for technological innovation.
The CMR was born from a long process of collaboration between The
University of Modena and Reggio Emilia and the Cassa di Risparmio
di Modena Foundation. Particularly, the Cassa di Risparmio di Modena
Foundation has been pursuing a double line of action: on the one hand
it has funded the Centre through a 13 million allocation for the real-
ization of structural, architectural, plant design works and equipment.
On the other hand it has also engineered and managed the complete
development and execution of the project. The ZPZ Partners team
and CDC took care of the building part and T.am.co and Ing.Ferrari
took care of the plant design part.
The CMR, opened in 2008. It is a 4-floor building on a total area of
4.000 square metres – that fulfills the highest normative requisites and
allows the culturing of cells in GMP conditions.
The ground floor is dedicated to offices, administration, warehouse
and services. There is also 5.500 liters of liquid-nitrogen tank with a
related distribution line and a carbon-dioxide exchange station with
a double flight of stairs, with a third emergency flight of stairs, with a
connected distribution line.
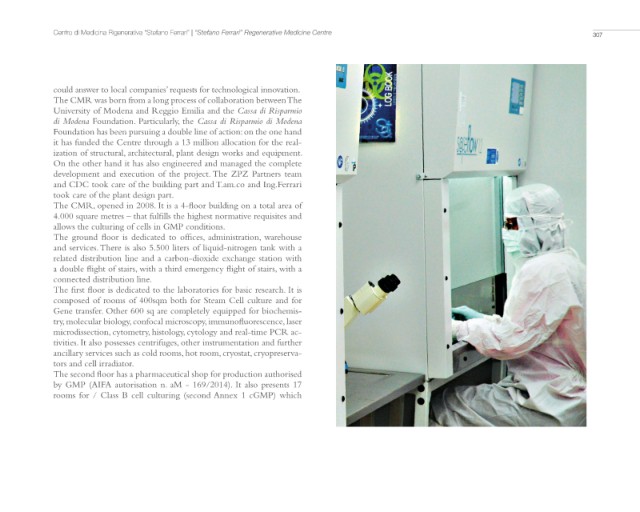
The first floor is dedicated to the laboratories for basic research. It is
composed of rooms of 400sqm both for Steam Cell culture and for
Gene transfer. Other 600 sq are completely equipped for biochemis-
try, molecular biology, confocal microscopy, immunofluorescence, laser
microdissection, cytometry, histology, cytology and real-time PCR ac-
tivities. It also possesses centrifuges, other instrumentation and further
ancillary services such as cold rooms, hot room, cryostat, cryopreserva-
tors and cell irradiator.
The second floor has a pharmaceutical shop for production authorised
by GMP (AIFA autorisation n. aM - 169/2014). It also presents 17
rooms for / Class B cell culturing (second Annex 1 cGMP) which